Cellulose, a natural plant polymer, is the most abundant biomass resource on earth. The usage of chemically processed cellulose started with the discovery of cellulose nitrate by Braconnot in France in 1833. Cellulose nitrate was mixed with about 30% camphor and plasticized to form the world’s first artificial plastic material, celluloid, about 150 years ago.
Cellulose acetate was developed in 1929 as a flame-resistant cellulose derivative due to the inflammability of celluloid. Until the advent of alternative petroleum plastics, cellulose acetate played an important role in industry as a plastic material for such things as photographic and cinematographic films, eyeglass frames, fibers, and packaging containers.
1. What is cellulose acetate?
Cellulose acetate is a biomass material produced by chemically modifying (esterifying) hydroxyl groups with acetic acids in cellulose materials derived from nonedible parts of plants, such as wood fibers and cotton. The structural formula of cellulose acetate is shown in Figure 1. The general physical properties of cellulose acetate are listed in Table 1.
Figure 1. Structural formula of cellulose acetate
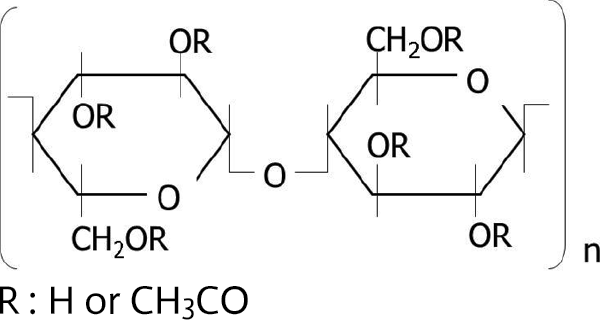
Table 1. General physical properties of cellulose acetate
| Item | Property | Unit |
|---|---|---|
| Shape | White and flaky | |
| Specific gravity (25/4℃) | 1.33 to 1.36 | - |
| Bulk density | 0.25 to 0.52 | kg/l |
| Glass transition temperature | 160 to 180 | ℃ |
| Melting point | 230 to 300 | ℃ |
| Specific heat | 1.34 | kJ/kg·K |
The properties of cellulose acetate are altered by a chemical modification of the hydroxyl groups in cellulose. The following materials are industrially manufactured in large quantities:
- (1)Cellulose triacetate (triacetate, TAC) with almost all hydroxyl groups modified with acetic acids
- (2)Cellulose diacetate (diacetate, DAC) with some hydroxyl groups unmodified)
Cellulose acetate generally has the following physical properties:
- ・Flame retardance with a high melting point (thermal softening point) of about 230°C
- ・Low electrical conductivity and insulating properties
- ・Moderate hydrophilicity
- ・Solvent and chemical resistance
- ・High resistance to ultraviolet rays
Currently, the proportion of biomass in cellulose acetate ranges from 50% to 60% because of the use of industrially produced acetic acid as a raw material. It is possible to achieve 100% by using acetic acids derived from biomaterials.
2. Biodegradability of cellulose acetate
Cellulose acetate is highly biodegradable in soil and seawater.[1-4] Generally speaking, cellulose acetate is degraded into cellulose and acetic acid through hydrolysis with water and biodegradation by esterase. Subsequently, the main chain of cellulose is biodegraded (cleaved/decomposed) by cellulase and is eventually converted into water and carbon dioxide. Plants bearing wood and cotton produce cellulose with water and carbon dioxide, thus forming the life cycle of cellulose acetate. (Figure 2)
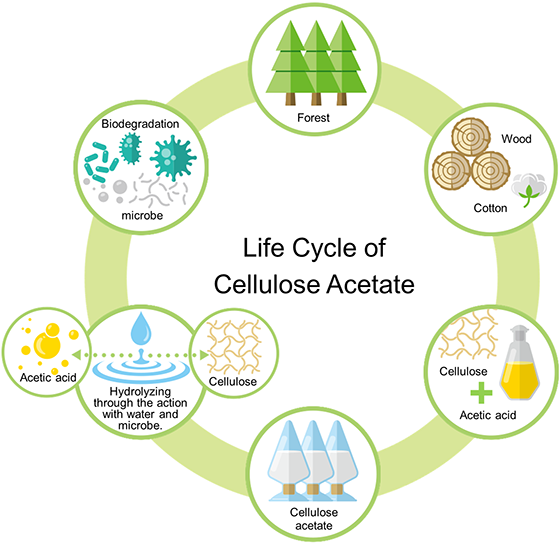
Figure 2. Cellulose acetate cycle
The biodegradability of cellulose acetate is as shown in Figure 3. General-purpose resins degrade over several decades to several hundreds of years, whereas cellulose acetate degrades much faster in nature (compost, soil, oceans, etc.).
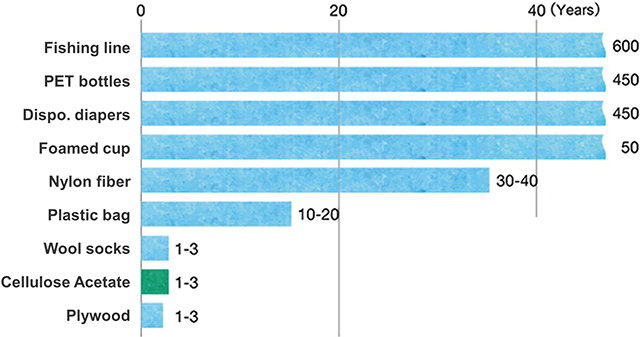
Figure 3. Degradation times of molded products in nature
(Based on data from previous reports[3, 5, 6])
Our product has been certified as a biomass (biomass plastic, BP) and biodegradable (green plastic, GP) plastic by the Japan BioPlastics Association (JBPA). Moreover, it has passed the biodegradability evaluation for the OK compost (INDUSTRIAL) certification of the internationally recognized certification body TÜV Austria (Figure 4) and has obtained its certification. In addition, it has also passed the biodegradability evaluation for the OK biodegradable Marine certification and has obtained the certification.

Figure 4. Biodegradation of cellulose acetate in industrial compost
3. Marine biodegradability of cellulose acetate
In recent years, marine plastic waste has been considered a major social problem. There are millions of tons of plastic waste floating in the oceans around the world every year due to improper disposal into oceans, unintentional discard of things like fishing gear, and spillage into oceans caused by wind and rain,[7] and this has negative effects on ecosystems. As described above, cellulose acetate is more biodegradable than petroleum resins and can biodegrade even in seawater.
By changing the molecular structure of cellulose acetate, we successfully developed a new form of it called CAFBLO™ with biodegradability nearly twice than that of conventional products in seawater, while maintaining its original functions and qualities. The biodegradability of various products in seawater is shown in Figure 5.
It is now understood that is possible to adjust the rate of biodegradation by controlling the molecular structure, and we hope that expanding our new product to a variety of applications in the future will help to solve the problem of marine plastic waste.
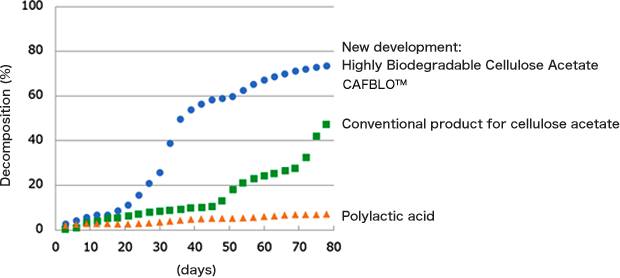
Figure 5. Marine biodegradability of cellulose acetate
(new and conventional products) and polylactic acid
(Based on the reference [6] and ASTM D6691,
with the biodegradation test results of the Chemicals Evaluation and Research Institute.)
4. Uses of cellulose acetate
The aforementioned biodegradable cellulose acetate can be used in the following two molding methods:
- (1)
Wet/dry molding after dissolution in a solvent
Cellulose acetate is soluble in certain solvents. DAC is used to create clothing fibers and filtration filters through dry spinning. TAC is also molded into a film or hollow thread shape to be used in such things as LCD optical films and filtration membranes. - (2)
Melt molding
Cellulose acetate has low plasticity, and thermoforming of it alone is difficult. Therefore, it is mixed with a plasticizer for melt molding. Traditionally, it has been used in applications that come into contact with the human body, such as eyeglass frames and combs. Our group company Daicel Miraizu Ltd. produces ACETY®, which utilizes a phthalate ester plasticizer, as injection molding pellets and CELBLEN® EC, which utilizes a non-phthalate plasticizer to comply with the environmental regulations starting to be introduced in Europe.
5. Future perspectives
Unlike other biodegradable plastics, cellulose acetate has been used since before the advent of petroleum resins. We believe that it has not been replaced by inexpensive resins because of its unique properties. Therefore, we believe that applications unique to cellulose acetate, as well as those shared in common with petroleum resins, should be further investigated.
At the same time, it should be applied more extensively as a biomass-derived biodegradable plastic to meet social needs. For that purpose, various thermoforming methods should be employed. Besides the development of new plasticizers and fluidity improvers, cellulose acetate should be compounded with other biodegradable plastics to improve the physical properties of resins.
Furthermore, policies should be implemented to raise the public awareness and understanding of biodegradable plastics. Correct understanding, proper use, and appropriate recycling of biodegradable plastics are critical for the realization of a circular economy. We believe that global environmental issues will be solved through the efforts of companies, government support, and public understanding. We will contribute to social and public well-being by developing a variety of materials, including cellulose acetate.
References
- [1]Sakai K, Yamauchi T, Nakasu F, Ohe T (1996). “Biodegradation of Cellulose Acetate by Neisseria sicca”. Bioscience, biotechnology, and biochemistry 60 (10): 1617-22.
- [2]Buchanan CM ,Gardner RM, Komarek RJ (1993). “Aerobic biodegradation of cellulose acetate” Journal of applied polymer science 47 (10): 1709-19.
- [3]WWF JAPAN WEB site https://www.wwf.or.jp/activities/basicinfo/3776.html
- [4]Studies on Chemical and Biological Degradability of Cellulose Acetate (abstract of doctoral thesis), Yoichiro Yamashita (2005), enrolled in the Graduate School Ph.D. Program, Advanced Chemistry of Functional Polymers, Department of Polymer Science and Engineering in 2001
- [5]U.S. National Park Service; Mote Marine Lab, Sarasota, FL and “Garbage In, Garbage Out,” Audubon magazine, Sept/Oct 1998
- [6]New Aspects of Cellulose Acetate Biodegradation, Dirk HÖLTER,Philippe LAPERSONNE, 2017_ST13
- [7]Annual reports on the Environment, the Sound Material-Cycle Society and Biodiversity in Japan, Ministry of Economy, Trade and Industry