HiSORAD™ is a co-processed excipient for orally disintegrating tablets, ODTs. It has a particularly high compactability.
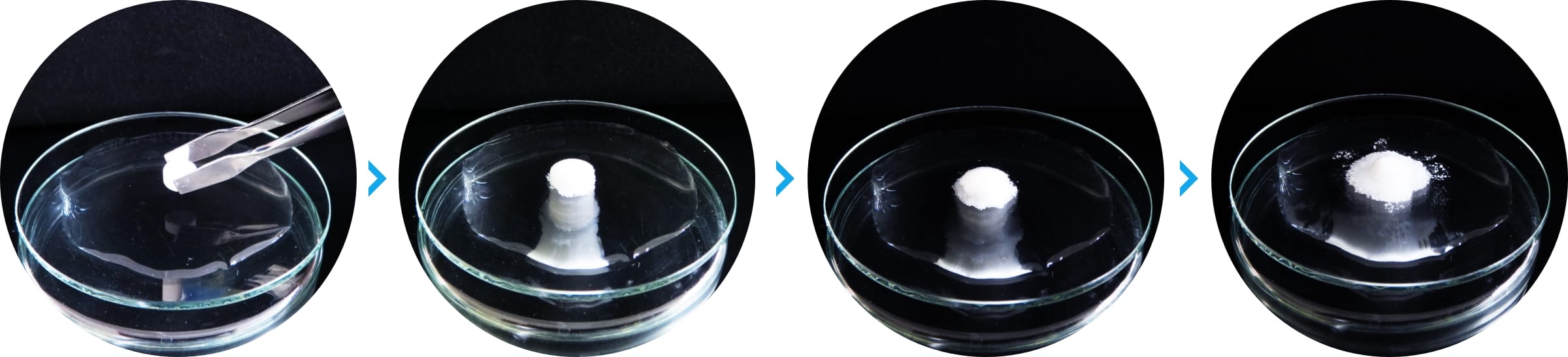
ODT with HiSORAD™ is quickly disrupted by contact with water.
HiSORAD™ Features
HiSORAD™ is a co-processed excipient for ODTs. It consists of "D-Mannitol", "Microcrystalline cellulose" and "Croscarmellose sodium". HiSORAD™ has excellent compactability so that high tablet hardness is possible at low compression force. Even with high API content and coated granules, it provides sufficient tablet hardness and rapid disintegration.
HiSORAD™ contributes to the simplification and efficiency of ODT formulation design.
- Excellent compactability for low compressible APIs and API pellets
- Well-balanced tablet property between OD time and hardness
- High API loading capacity
- Simple composition
Regulation
- All components of HiSORAD™ are included in JP, USP and EP.
- US-DMF is available for HiSORAD™.
Powder properties
The particles of HiSORAD™ has non-spherical shape since it is processed by our unique granulation method. This special shape contributes to good content uniformity.
SEM photograph of HiSORAD™

Powder Properties
| Items | HSR-D03 |
|---|---|
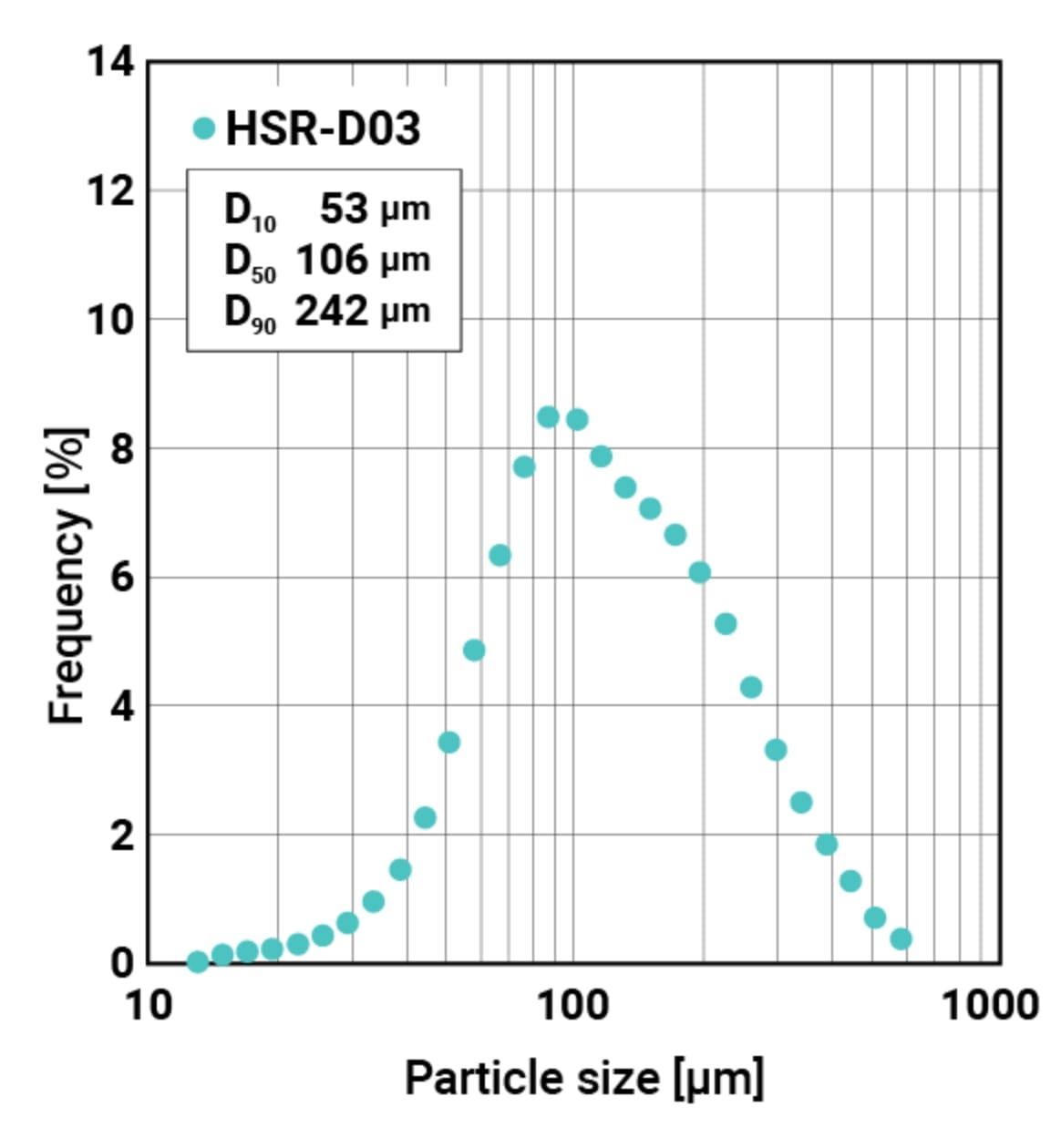
| Mean particle size※1 | (Median) 106 μm |
| Density (Loose) | 0.35 g/cm3 |
| Density (Tapping) | 0.49 g/cm3 |
| Angle of repose | 41 ° |
| Orifice diameter | 6.3 mm |
| Water content | 2.4 wt% |
※1 Measured by dry laser diffraction/scattering instrument
Particle size distribution
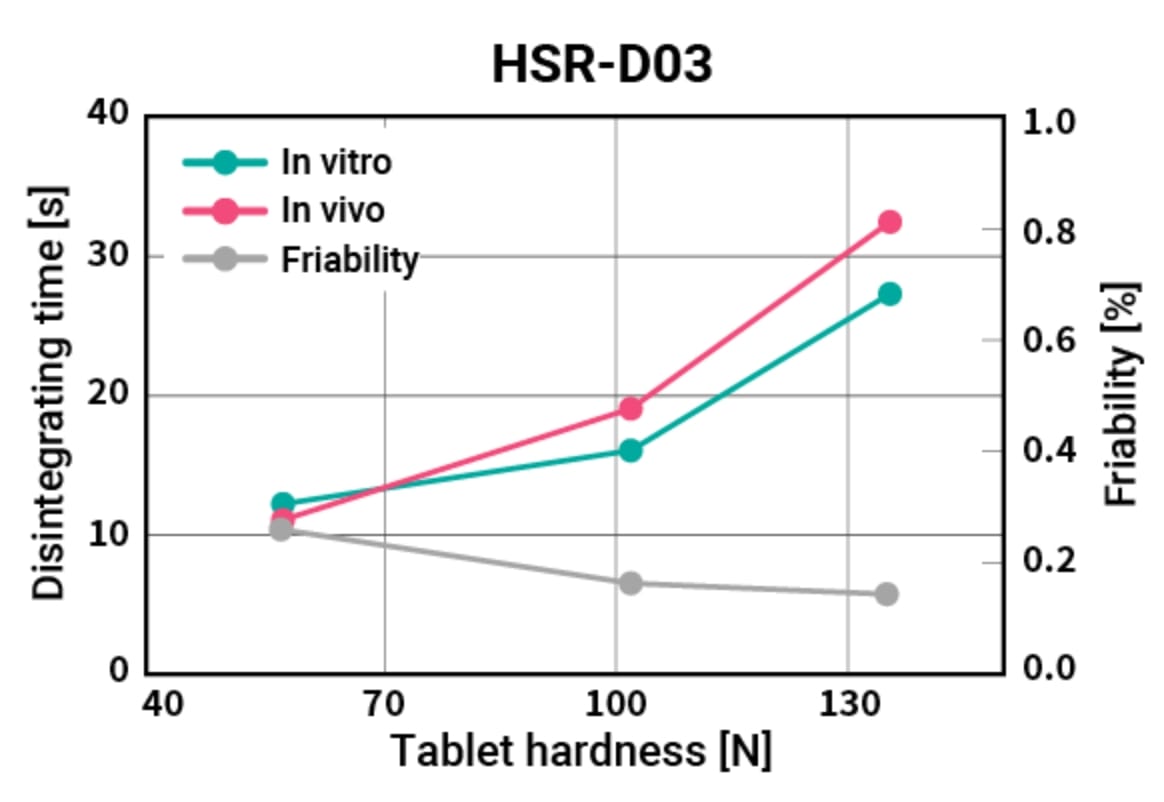
Placebo tablet performance
Placebo tablets with HiSORAD™ are compatible with adequate tablet hardness and rapid disintegration. Compression force shows a higher tablet hardness even in the low region of compression force.
Tablet performance of placebo ODTs
HiSORAD™ maintains good tablet performance even with high dose API.
Examples of high-dose ODT
Other applications
- Basic performance of HiSORAD™
- Applicability of co-processed excipients to continuous manufacturing: Dry granulation
- Applicability of co-processed excipients to continuous manufacturing: Direct compression
HiSORAD™ contributes to the simplification and efficiency of ODT formulation design.
Leaflet on HiSORAD™ is available.


