Formulation technology
GRANFILLER-D™ and HiSORAD™ are co-processed excipients for orally disintegrating tablets (ODTs).
Both products provide well-balanced property between OD time and hardness.

- Well-balanced tablet property between OD time and hardness
- High API loading capacity
- Excellent content uniformity
- Compatible with various excipients

- Excellent compactability for low compressible APIs and API pellets
- Well-balanced tablet property between OD time and hardness
- High API loading capacity
- Simple composition
What are ODTs?
Orally disintegrating tablets, ODTs can disintegrate easily with a small amount of water such as saliva, and there is no need to swallow the whole tablet as solid form with water. Therefore, ODTs are a safe and reliable dosage form that is easy to swallow for patients with the risk of aspiration. From another perspective, ODTs offer the convenience of taking the medication quickly and easily in any situation where water is not readily available, such as when going out or in an emergency. Moreover, ODTs can be applied to patients with limited water intake.

Performance Requirements for ODTs
ODTs are required to have sufficient tablet strength for handling until they are taken. At the same time, they must disintegrate quickly on contact with saliva in the oral cavity. Advanced technologies are necessary to formulate ODTs because they must strike a balance between the conflict performances.

The solution by DAICEL
Conventional ODTs sometime have insufficient tablet hardness to ensure rapid disintegration, however, ODTs with GRANFILLER-D™ or HiSORAD™ have the tablet hardness of 100 N under the disintegration time of less than 20 seconds. They can achieve both functions.
Technologies for ODTs
In the 1980s, the first generation of ODTs was developed using lyophilization. From that time ODTs became popular and various technologies were generated such as molding and dry tableting. While lyophilization and molding require particular facilities, the dry tableting method require conventional manufacturing and packaging machineries only. Because it is the simplest and most cost-effective ODT manufacturing technique, is now used by many pharmaceutical companies.
Recently, co-processed excipients for direct compression have been developed to provide functions and better powder property of the excipients. Functions such as compactability and disintegratability affect ODT quality. The powder property such as particle size and size distribution obviously affect the stability of the direct compression process. DAICEL also launched the CPEs, GRANFILLER-D™ in 2014 and HiSORAD™ in 2020.
What is the direct compression?
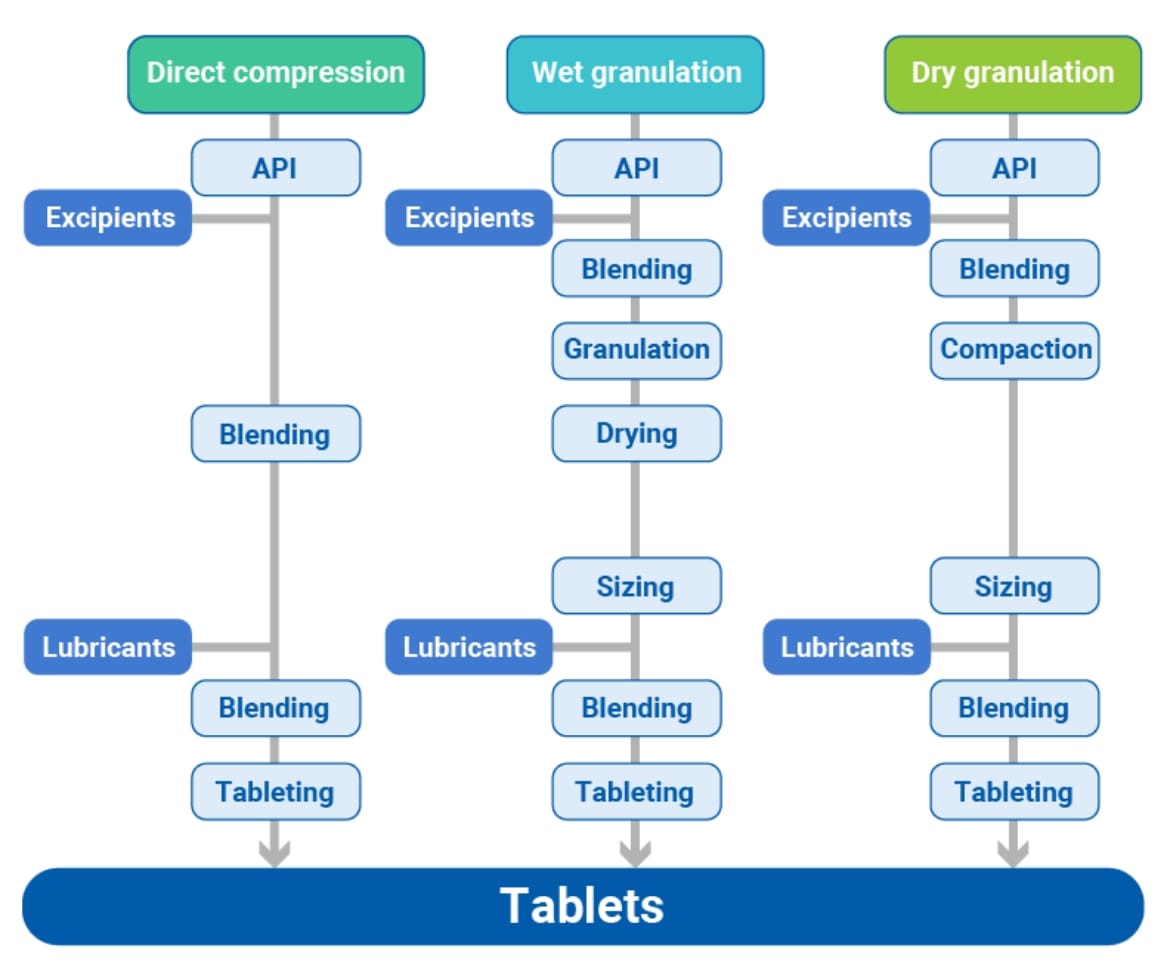
There are two major conventional processes to prepare tablets: compression method and molding method.
The former can be subdivided into direct compression, wet granulation and dry granulation. The purpose of granulation is to convert mixture of API and excipient into granules which flow well into equipment, maintain content uniformity, and can be compressed into tablets without tableting disorders. However, wet or dry granulation processes become complex and typically cost consuming. Direct compression is a method involves only blending and tableting processes. It is the preferred way of manufacturing tablets, since it is the simplest process, shortens production time, and reduces costs. However, there are still challenges to overcome, as powder property of raw materials directly affect to the tablet quality. There is concern powder segregation, tableting disorders, fluctuations in tablet weight etc.
- Pros.
-
- Can handle moisture-sensitive APIs
- Simple manufacturing process
- Cost-effective
- Cons.
-
- Tableting disorder
- Tablet weight fluctuations
- Variation of content uniformity
The solution by DAICEL
Prevention of segregation
The particles of GRANFILLER-D™ and HiSORAD™ have non-spherical shape since it is processed by DAICEL’s unique granulation method. The shape is considered to contribute to keeping ordered mixture that prevents segregation. GRANFILLER-D™ and HiSORAD™ can be applicable to wide range of particles from micronized APIs to API pellets, maintaining the content uniformity.

Non-spherical particle shape
What are the co-processed excipients?
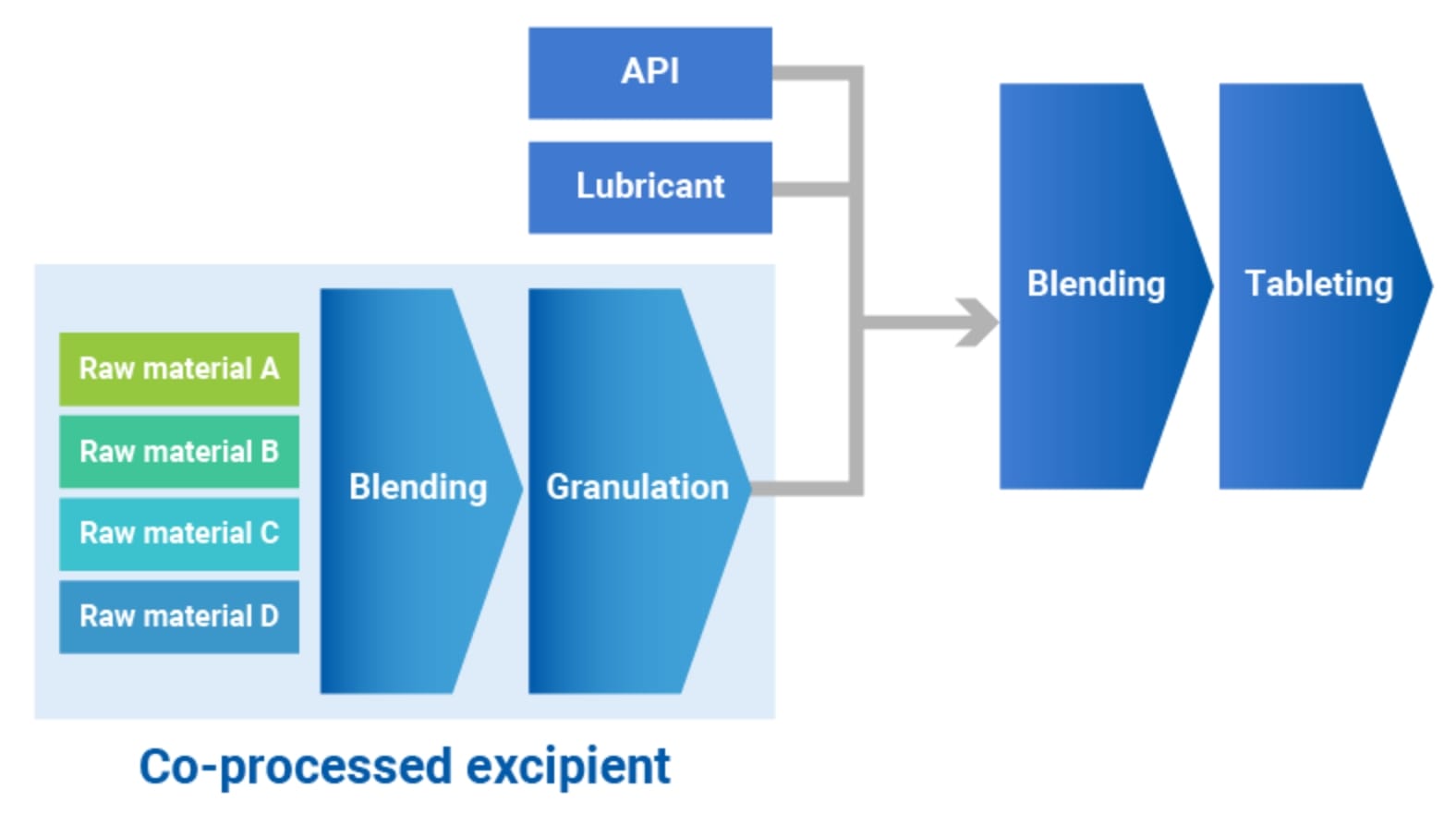
Co-processed excipients are excipients produced by processing several raw materials. Since the particles are compounded different excipients with different features and processed, co-processed excipients provide new functions that a single excipient cannot achieve.
It usually takes considerable time for manufacturers to develop formulations, because it is required to select appropriate excipients for each API. Furthermore, recent APIs require extra processing steps to improve stability or bio-availability. There are a multitude of tasks for developments. Under these circumstances, co-processed excipients can be time-saving tool in the development phase. In commercial production phase, the co-processed excipients enable to eliminate granulation process and manufactures conduct simple production process by mixing and direct compression alone.
Advantages of co-processed excipients
- Development phase
- Reduction of time for selection and evaluation of excipients
- Commercial production phase
-
Eliminating granulation process
Simple production process by mixing and direct compression alone
The solution by DAICEL
GRANFILLER-D™ and HiSORAD™ are also characterized as CPEs. They are manufactured by mixing several pharmaceutical excipients in an optimized composition and processing with a unique granulation method according to the intended purpose. They are designed to maximize the characteristics of each ingredient and have functions and powder properties suitable for the manufacture of ODTs.


What GRANFILLER-D™ and HiSORAD™ can do for you
GRANFILLER-D™ and HiSORAD™ contribute to the development of high-performance ODTs. Detailed technical information can be found on the respective product pages or in the white paper.
Preparetion of bitterness masking ODTs
In this paper, we report the preparation of an ODT including a β-Cyclodextrin (β-CD)-masked Mitiglinide Ca (MIT). The ODT was prepared with the β-CD/MIT complex and GRANFILLER-D. It showed excellent tablet hardness and disintegration even when high content of β-CD/MIT complex was applied. Furthermore, the ODT showed similar disintegration time with higher tablet hardness than the originator's drug.
Applicability of co-processed excipients to continuous manufacturing: Direct compression
In Continuous Manufacturing (CM), minimizing lot-to-lot differences in raw materials is important for stabilizing product quality. The application of co-processed excipients may be useful in resolving this issue because they allow multiple excipients to be treated like a single excipient. In this report, HiSORAD™ was applied to the CM and evaluated. Compared to the physical mixture, HiSORAD™ performed better in all evaluations and meet the set quality target product profile.
Preparetion of mini ODTs
In this article, we report the development of mini ODTs which shape are suitable for pediatric formulations. The sample using GRANFILLER-D or HiSORAD showed good tablet performances with the coefficient of variation for tablet weight ≤ 1.2%. Both tablets achieved disintegration time of
