Ultra reduction using sunlight. It decomposes CO₂ semi-permanently and continuously converts it into raw materials through exposure to sunlight alone.
Towards a new carbon negative future.
Scroll

Ultra reduction using sunlight
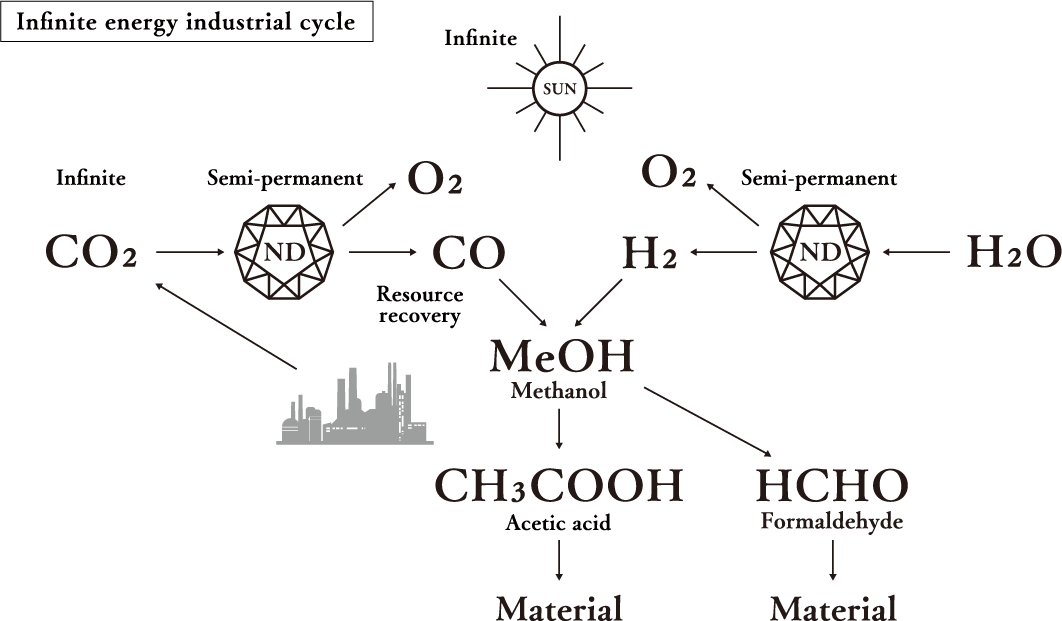
Most existing CO₂ reduction technologies need large amounts of electric power to decompose CO₂ and also generate CO₂ when producing that electric power. Daicel’s ultra reduction using sunlight continuously decomposes CO₂ into carbon monoxide and oxygen with high efficiency using hydrated electrons generated in the surrounding space by exposing nanodiamonds to sunlight alone. In addition, since nanodiamonds do not deteriorate, the reaction is semi-permanent. Furthermore, it is also possible to decompose H₂O into hydrogen and oxygen. If methanol, for example, is synthesized from the generated hydrogen and carbon monoxide, it can be reused as a raw material.
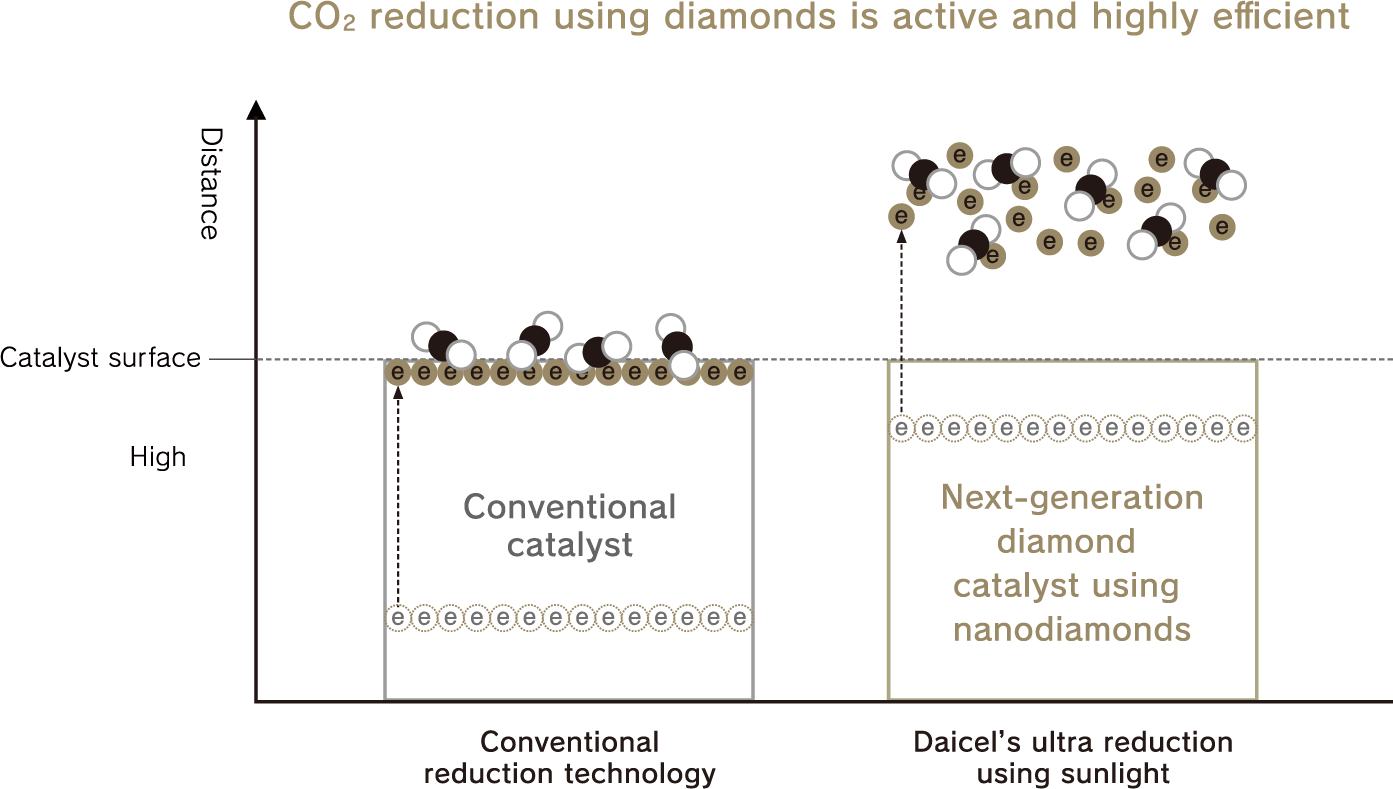
The ultra reducing using sunlight nanodiamond solution is 3D reduction.
Reduction with existing technologies occurs only on the surface of a solid catalyst; however, with ultra reduction using sunlight, hydrated electrons are emitted into the surrounding space, realizing a highly efficient reduction reaction in three dimensions rather than two dimensions.
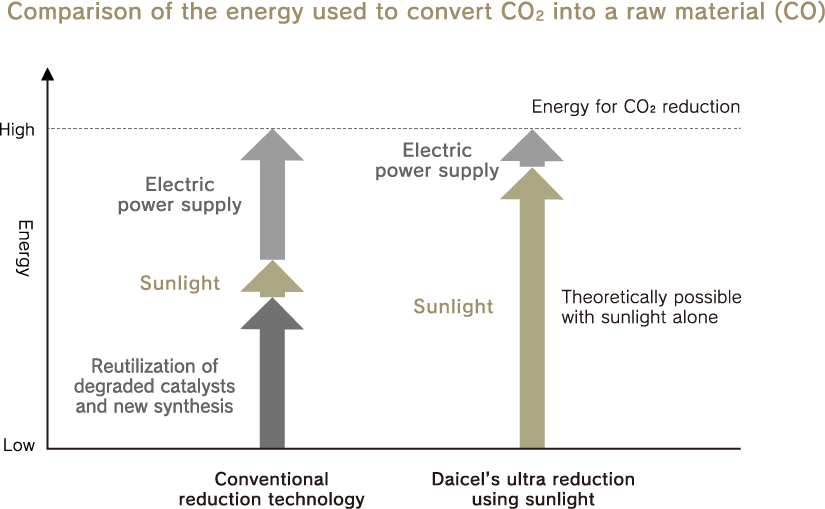
The only energy needed is sunlight.
A one-of-a-kind carbon-negative technology.
Existing CO₂ reduction technologies require electricity to enable the adsorption of CO₂ in a catalyst and reaction with electrons, and CO₂ is generated when electricity is used. On the other hand, ultra reduction using sunlight requires minimal artificial energy because electrons in nanodiamonds are emitted into the surrounding space just through exposure to sunlight. Furthermore, since nanodiamonds are chemically stable and do not deteriorate, they decompose CO₂ semi-permanently.